- Applications
- Oral Gavage
- IV Drug Infusion
- Mouse Intermittent
- Mouse Continuous Infusion
- Mouse Continuous Infusion Plus Blood Sampling
- Mouse Two Channel Infusion
- Rat Intermittent
- Rat Continuous Infusion
- Rat Intermittent Infusion and Blood Sampling
- Rat Continuous Infusion Plus Blood Sampling
- Rat IV Bolus
- Rat Two-Channel Infusion
- Rat Infusion and Whole Body Plethysmography
- Rat GLP Infusion Toxicology Studies
- Large Animal Continuous Ambulatory Infusion
- IV Self-Administration
- Blood Sampling
- Glucose Clamp
- Bile Sampling
- Products
- Education
- Resources
- About
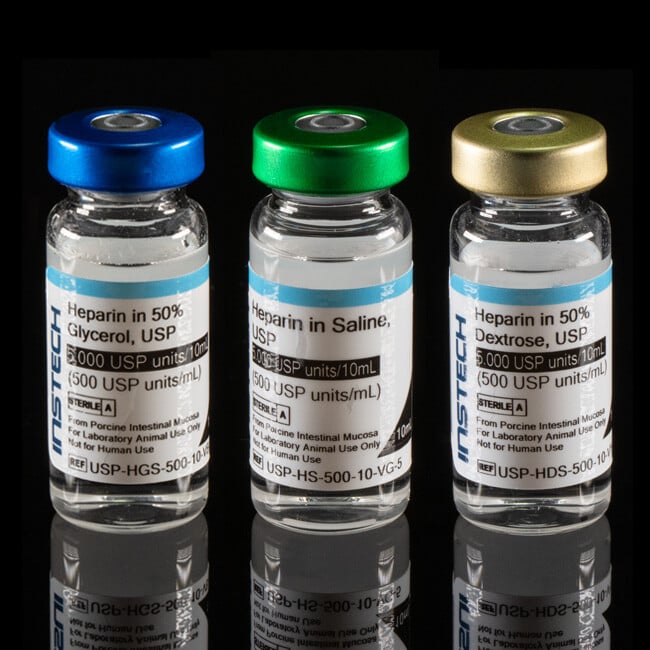
Catheter Flush and Lock Solutions
Pharmaceutical-Grade Flush and Lock Solutions for Research
Are you putting your laboratory animals and research at risk by filling implanted catheters with solutions that are not pharmaceutical-grade or, worse, not even sterile?
Our line of sterile solutions are made with USP heparin sodium and other pharmaceutical-grade ingredients following the USP <797> standard for compounding sterile preparations.
NIH's Office of Laboratory Animal Welfare states: OLAW and USDA agree that pharmaceutical-grade substances, when available, must be used to avoid toxicity or side effects that may threaten the health and welfare of vertebrate animals and / or interfere with the interpretation of research results. See this guidance document for more information.
AAALAC states: Whenever possible, pharmaceutical-grade compounds must be used [for clinical use]. If available, and suitable, pharmaceutical-grade compounds are preferred [for research use].
These solutions are not approved for human use. They are for laboratory animal research only.